missing translation for 'onlineSavingsMsg'
Learn More
Learn More
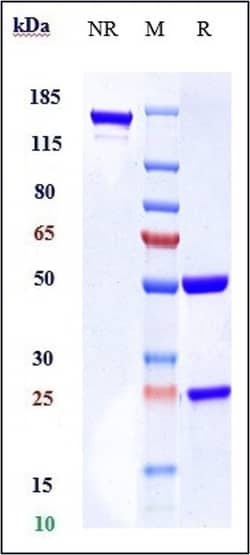
Invitrogen™ Mogamulizumab Recombinant Monoclonal Antibody


Recombinant Monoclonal Antibody
£397.00 - £1002.00
Specifications
| Antigen | Mogamulizumab Humanized |
|---|---|
| Concentration | 1 mg/mL |
| Content And Storage | -20°C, Avoid Freeze/Thaw Cycles |
| Applications | ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance |
| Classification | Recombinant Monoclonal |
| Product Code | Brand | Quantity | Price | Quantity & Availability | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product Code | Brand | Quantity | Price | Quantity & Availability | |||||
30284668
 |
Invitrogen™
MA558471 |
100 μg |
£397.00
100µg |
Please sign in to purchase this item. Need a web account? Register with us today! | |||||
|
30282828
|
Invitrogen™
MA558472 |
1 mg |
£1002.00
1mg |
Please sign in to purchase this item. Need a web account? Register with us today! | |||||
Description
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
Mogamulizumab is a humanized monoclonal antibody (mAb) directed against CC chemokine receptor 4 (CCR4) for the treatment of Mycosis Fungoides (MF) and Sezary Syndrome (SS), the most common subtypes of cutaneous T-cell lymphoma. Cutaneous T-cell lymphomas occur when certain white blood cells, called T cells, become cancerous; these cancers typically affect the skin, causing various types of skin lesions 8. On August 8 2018, the U. S. Food and pharmaceutical Administration (FDA) approved mogamulizumab injection (also known as Poteligeo) for intravenous use for the treatment of adult patients with relapsed or refractory mycosis fungoides (MF) or Sezary syndrome (SS) after at least one prior systemic therapy 7. Mogamulizumab is derived from Kyowa Hakko Kirin's POTELLIGENT (™) technology, which produces antibodies with enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) activity. Approval in Japan was granted on April 30 2012 by the Japanese Ministry of Health, Labor and Welfare for patients with relapsed or refractory CCR4-positive adult T-cell leukemia-lymphoma 2.Specifications
| Mogamulizumab Humanized | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Recombinant Monoclonal | |
| Lyophilized | |
| IgG1 | |
| Human | |
| AMG-761; KW-0761 | |
| Antibody |
| 1 mg/mL | |
| ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance | |
| Unconjugated | |
| Human | |
| RUO | |
| 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 | |
| Primary | |
| Protein A |
Spot an opportunity for improvement?Share a Content Correction
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title