missing translation for 'onlineSavingsMsg'
Learn More
Learn More
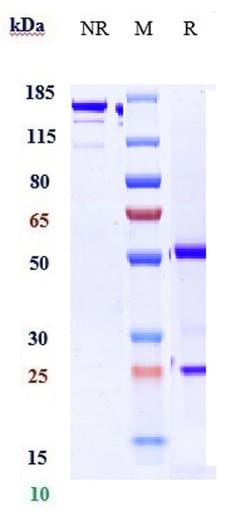
Invitrogen™ Golimumab Recombinant Monoclonal Antibody


Recombinant Monoclonal Antibody
£397.00 - £1002.00
Specifications
| Antigen | Golimumab |
|---|---|
| Concentration | 1 mg/mL |
| Content And Storage | -20°C, Avoid Freeze/Thaw Cycles |
| Applications | ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance |
| Classification | Recombinant Monoclonal |
| Product Code | Brand | Quantity | Price | Quantity & Availability | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product Code | Brand | Quantity | Price | Quantity & Availability | |||||
30283393
 |
Invitrogen™
MA559395 |
100 μg |
£397.00
100µg |
Please sign in to purchase this item. Need a web account? Register with us today! | |||||
|
30283436
|
Invitrogen™
MA559396 |
1 mg |
£1002.00
1mg |
Please sign in to purchase this item. Need a web account? Register with us today! | |||||
Description
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
Golimumab is a human IgG1kappa monoclonal antibody derived from immunizing genetically engineered mice with human TNFalpha. Golimumab binds and inhibits soluble and transmembrane human TNFalpha. Increased TNFalpha is associated with chronic inflammation. Thus golimumab is indicated for use in adults (i) as an adjunct to methotrexate treatment in patients with moderate to severe active rheumatoid arthritis (RA), (ii) alone or as an adjunct to methotrexate treatment in patients with active psoriatic arthritis (PsA), (iii) as a single agent in patients with active ankylosing spondylitis (AS), and (iv) as a single agent in patients with moderate to severe ulcerative colitis (UC) who require chronic steroids or have experienced intolerance or only a partial response to previous medications. In the U. S. and Canada, golimumab is marketed under the brand name Simponi™. The FDA label includes a black box warning of serious infections and malignancy. Additionally in children and adolescents taking golimumab, there have been lymphoma and other malignancies observed. As a human monoclonal antibody, golimumab binds and inhibits soluble and transmembrane human TNFalpha. Inhibition of TNFalpha prevents it binding to its receptors, which prevents both leukocyte infiltration through prevention of cell adhesion proteins such as E-selectin, ICAM-1 and VCAM-1, and pro-inflammatory cytokine secretion such as IL-6, IL-8, G-CSF and GM-CSF in vitro. Consequently, in patients with chronic inflammatory conditions, decreases in ICAM-1 and IL-6 as well as C-reactive protein (CRP), matrix metalloproteinase 3 (MMP-3), and vascular endothelial growth factor (VEGF) were observed.Specifications
| Golimumab | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Recombinant Monoclonal | |
| Lyophilized | |
| IgG1 | |
| Human | |
| CNTO 148; Simponi | |
| Antibody |
| 1 mg/mL | |
| ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance | |
| Unconjugated | |
| Human | |
| RUO | |
| 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 | |
| Primary | |
| Protein A |
Spot an opportunity for improvement?Share a Content Correction
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title