missing translation for 'onlineSavingsMsg'
Learn More
Learn More
Invitrogen™ Daclizumab Recombinant Monoclonal Antibody


Recombinant Monoclonal Antibody
£397.00 - £1002.00
Specifications
| Antigen | Daclizumab Humanized |
|---|---|
| Concentration | 1 mg/mL |
| Content And Storage | -20°C, Avoid Freeze/Thaw Cycles |
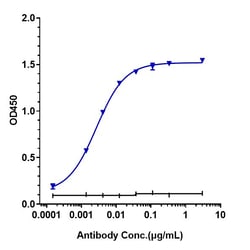
| Applications | ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance |
| Classification | Recombinant Monoclonal |
| Product Code | Brand | Quantity | Price | Quantity & Availability | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product Code | Brand | Quantity | Price | Quantity & Availability | |||||
30283004
 |
Invitrogen™
MA558663 |
100 μg |
£397.00
100µg |
Please sign in to purchase this item. Need a web account? Register with us today! | |||||
|
30283917
|
Invitrogen™
MA558664 |
1 mg |
£1002.00
1mg |
Please sign in to purchase this item. Need a web account? Register with us today! | |||||
Description
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
Humanized IgG1 Mab that binds to the human interleukin-2 receptor (anti-Tac or anti-CD25). Daclizumab is a composite of human (90%) and murine (10%) antibody sequences. The human sequences were derived from the constant domains of human IgG1 and the variable framework regions of the Eu myeloma antibody. The murine sequences were derived from the complementarity-determining regions of a murine anti-Tac antibody. On 22 April 2008, Roche Registration Limited chose to voluntarily withdraw the marketing authorization for their product Zenapax (daclizumab), as indicated for the prophylaxis of acute organ rejection in de novo allogeneic renal transplantation and used concomitantly with an immunosuppressive regimen like cyclosporine and corticosteroids in patients who are not immunized, for commercial reasons and confirmed that this decision was not related to any safety concerns associated with the use of Zenapax (daclizumab) 2. Regardless of the withdrawal of Zenapax, Biogen and Abbvie's Zinbryta (daclizumab), as indicated for the treatment of adult patients with relapsing forms of multiple sclerosis, was approved for use by the FDA in 2016.Specifications
| Daclizumab Humanized | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Recombinant Monoclonal | |
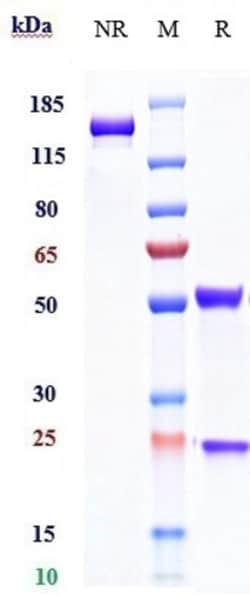
| Lyophilized | |
| IgG1 | |
| Human | |
| BIIB019; DAC HYP | |
| Antibody |
| 1 mg/mL | |
| ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance | |
| Unconjugated | |
| Human | |
| RUO | |
| 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 | |
| Primary | |
| Protein A |
Spot an opportunity for improvement?Share a Content Correction
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title