missing translation for 'onlineSavingsMsg'
Learn More
Learn More
Invitrogen™ Canakinumab Recombinant Monoclonal Antibody


Recombinant Monoclonal Antibody
£397.00 - £1002.00
Specifications
| Antigen | Canakinumab |
|---|---|
| Concentration | 1 mg/mL |
| Content And Storage | -20°C, Avoid Freeze/Thaw Cycles |
| Applications | ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance |
| Classification | Recombinant Monoclonal |
| Product Code | Brand | Quantity | Price | Quantity & Availability | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product Code | Brand | Quantity | Price | Quantity & Availability | |||||
30283365
 |
Invitrogen™
MA558961 |
100 μg |
£397.00
100µg |
Please sign in to purchase this item. Need a web account? Register with us today! | |||||
|
30283086
|
Invitrogen™
MA558962 |
1 mg |
£1002.00
1mg |
Please sign in to purchase this item. Need a web account? Register with us today! | |||||
Description
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
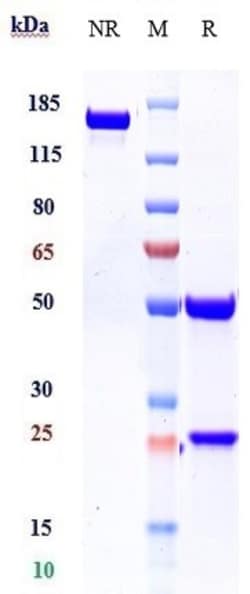
Canakinumab is a recombinant, human anti-human-IL-1beta monoclonal antibody that belongs to the IgG1/kappa isotype subclass. It is expressed in a murine Sp2/0-Ag14 cell line and comprised of two 447- (or 448-) residue heavy chains and two 214-residue light chains, with a molecular mass of 145157 Daltons when deglycosylated. Both heavy chains of canakinumab contain oligosaccharide chains linked to the protein backbone at asparagine 298 (Asn298). Canakinumab binds to human IL-1beta and neutralizes its inflammatory activity by blocking its interaction with IL-1 receptors, but it does not bind IL-1alpha or IL-1 receptor antagonist (IL-1ra). Canakinumab is marketed under the brand name Ilaris and indicated for patients 4 years of age and older to treat Familial Cold Autoinflammatory Syndrome (FCAS) and Muckle-Wells Syndrome (MWS), which are both part of the Cryopyrin-Associated Periodic Syndromes (CAPS) as well as for patients 2 years of age and older to treat systemic juvenile idiopathic arthritis (SJIA). Clinical trials have established the administration of canakinumab every 2 weeks to be safe and effective, offering a considerable advantage over the existing treatment with the human IL-1 receptor antagonist, anakinra, which must be injected daily and which is often poorly tolerated by patients.Specifications
| Canakinumab | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Recombinant Monoclonal | |
| Lyophilized | |
| IgG1 κ | |
| Human | |
| ACZ885 | |
| Antibody |
| 1 mg/mL | |
| ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance | |
| Unconjugated | |
| Human | |
| RUO | |
| 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 | |
| Primary | |
| Protein A |
Spot an opportunity for improvement?Share a Content Correction
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title