Advanced Cell Culture Solutions for Cell Therapy
Ensure cell therapy safety with our consistent, high-quality raw materials, from premium (Pre-GMP) grade to GMP, and from discovery to clinic.
Product Benefits
CMC Process Solutions

Related Products
Product Code 31452286
Product Code 31453495
Product Code 31451885
Product Code 31452442
Product Code 30214564
Product Code 30214600
Product Code 17876615
Product Code 31451800
Related Videos
Looking for a Resilient Supplier for Your CGT Development?
See how ACRO can help you.
iPSC-Derived Cell Therapy: Where Are We Now, and What’s Next
Next-generation IPSC cell therapy.
Related Documentation
Frequently Asked Questions
We source materials from qualified suppliers, conduct annual risk assessments, and perform visual and documentary checks (e.g., CoA, CoO, TSE/BSE declarations and animal-free statements). Key materials are validated before use.
Adopting GMP grade raw materials in early development can streamline the transition into later development stages.
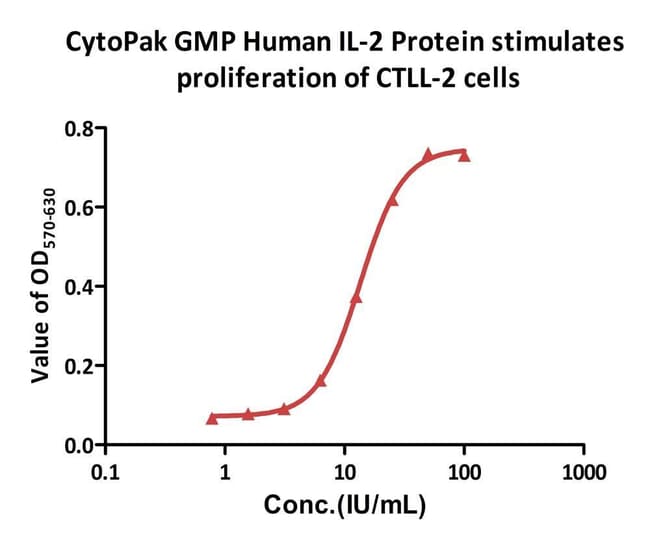
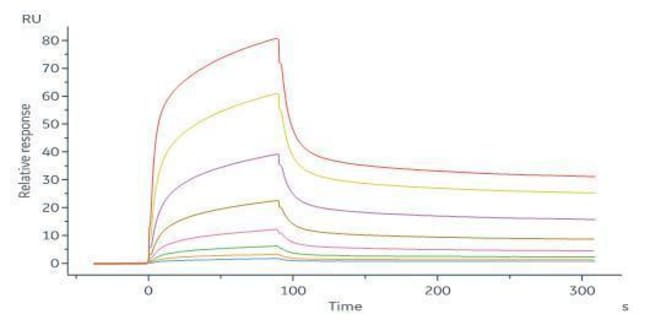
Biological reagents like cytokines require rigorous validation through multiple batches to ensure consistency. Manufacturing under strict GMP controls, documented SOPs, and trained personnel ensure reliability. We assess multi-batch data and compare new batches to standards, maintaining lot-to-lot biological activity variability within 10-15%.
Overcoming Challenges from Discovery to the Clinic
Overcoming challenges from discovery to the clinic with innovation tools and solutions.